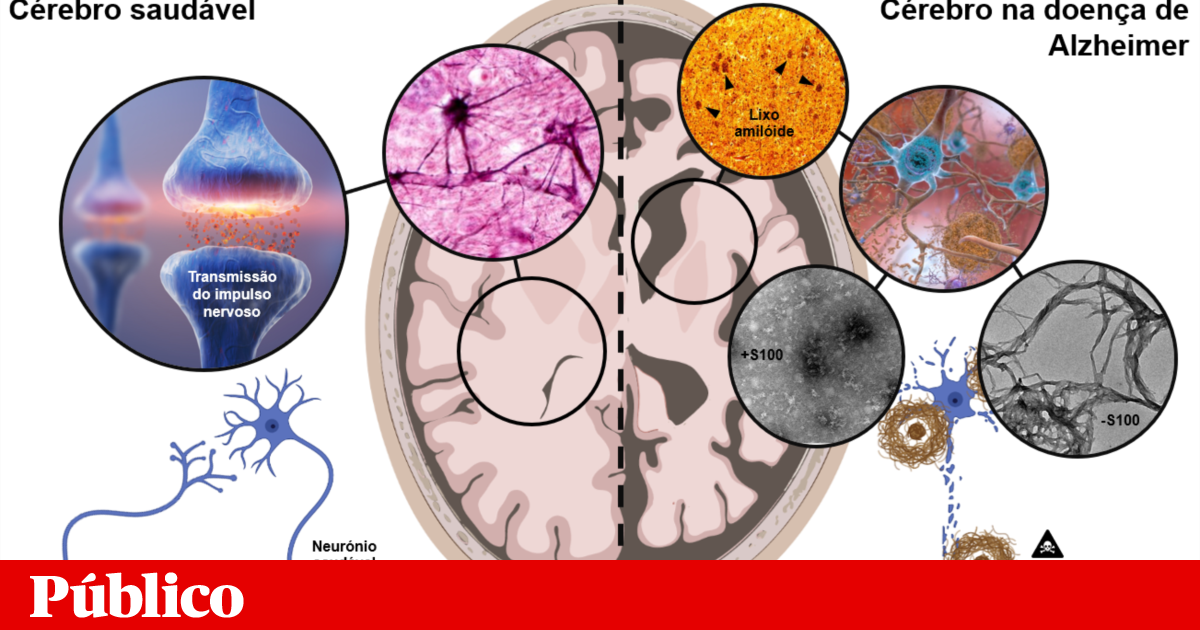
Proteins are large molecules that perform many functions in living organisms, and in order to function properly, they need to acquire a specific three-dimensional shape. However, some proteins can be mistaken for folding, binding, and forming a type of toxic waste called amyloid, whose microscopic structure resembles a tangle of hair. Accumulating in cells, tissues and organs, these fibers contribute to the development of various diseases. An example is Alzheimer’s disease, which is the most common cause of dementia in the world.
In this neurodegenerative disease, two proteins — amyloid beta and tau — aggregate to make this junk inside and outside neurons, a type of brain cell.
However, organisms have evolved in order to develop mechanisms to prevent these phenomena from occurring in abundance. In fact, another class of proteins, called “molecular chaperones,” whose role is to prevent the formation of amyloid waste products and to ensure that proteins acquire their correct structure. Although many of these proteins exist, the number of chaperones identified as being able to act on amyloid beta for Alzheimer’s disease remains limited.
This is where my PhD work fits. Recently, an abundant protein in the brain called ‘S100B’ – so far only associated with inflammatory processes – has been described as a beta-amyloid cofactor. Indeed, S100B was able to delay and prevent the formation of amyloid beta fibrils, protecting neurons from its toxic effects.
doctor
However, there are other members of the brain S100 protein family, very similar to S100B, whose potential neuroprotective effects are not known. My work aims to study how this family of proteins regulate the formation of beta-amyloid aggregates, using approaches from genetic engineering, structural biochemistry, molecular biology and even animal models.
Until then, my research confirmed that, in fact, other relatives of S100B are also able to inhibit the formation of amyloid-beta protein fibrils, in some cases more effectively. Furthermore, we realized that the mode of action of these proteins allows the formation of a very specific and highly toxic type of amyloid waste to reduce them, which may underlie the beneficial effects of S100 proteins.
While not a cure in and of itself, understanding the brain’s mechanisms to prevent the formation of amyloid fibers in the early stages of Alzheimer’s disease could inspire promising therapies. Cumulatively, this knowledge may help identify diagnostic biomarkers that allow a patient to know whether they will develop pathology even before clinical symptoms appear.
The author writes according to the new spelling convention
PhD student in Biochemistry at the Faculty of Sciences of the University of Lisbon

“Wannabe internet buff. Future teen idol. Hardcore zombie guru. Gamer. Avid creator. Entrepreneur. Bacon ninja.”