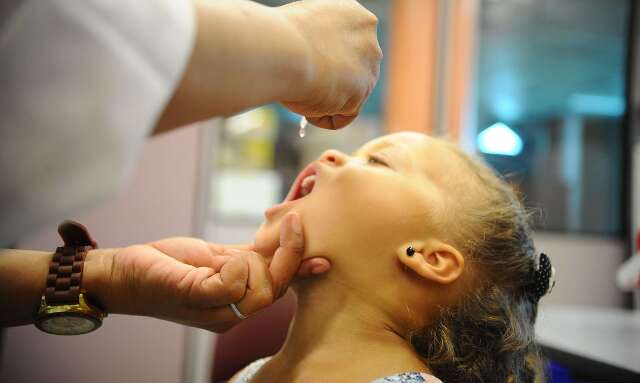
The polio vaccination was able to eradicate the disease from entire continents, it developed with the advent of more advanced technologies and has been out of reach of Brazilian children for more than 30 years. However, the drop in vaccine coverage has been a concern for researchers in the region, who ensure that the full vaccination schedule proposed by the National Immunization Program (PNI) protects children from polio and virtually eliminates the possibility of adverse events. .serious.
In Brazil, all children should receive the inactivated polio vaccine (VIP) at 2 months, 4 months, and 6 months of age. This vaccine has been injected since 2012, and its use has made polio immunization safer, because the vaccine content is inactivated “dead” virus, eliminating any risk of virus replication after vaccination.
After being immunized with these three doses, Brazilian children should receive the famous drops of the oral polio vaccine (VOP), at 15 months and 4 years of age, as a booster in the immunization. The practical application of the vaccine also makes it used in mass vaccination campaigns, such as those carried out by the Ministry of healthbetween August 8 and September 30.
When the oral vaccine is given without first using the inactivated vaccine, there is an extremely rare risk that the attenuated virus in the droplets will cause adverse events, such as paralysis itself, explains the Vice President of the Brazilian Society for Immunization (SBIm), Isabella Blalay. The doctor remembers that cases like this have also been eradicated in Brazil because the vaccination schedule now contains three doses of the inactivated vaccine.
“Since Brazil started vaccination with the first three doses [em 2012]No more cases of polio vaccine were seen. Prior to that, the incidence was one case for every 2.450 million doses applied,” explains Isabella Balalai. The most important thing is that all the children who took the VIP, because they will be protected from the vaccine virus, they will be protected when they take the OPV and they will be protected in case of any outbreak of polio virus.”
search in Para
By excluding that the case of a child with acute flaccid paralysis in Parra could be poliomyelitis caused by wild-type virus, the Ministry of health He noted the possibility that the symptoms were caused by improper vaccination. “There is no record in the child’s vaccination book of inactivated polio vaccine (VIP), which must be given before the oral polio vaccine (drops). In general, OPV is well-tolerated, rarely associated with a risk of infection. It is indicated that the risk of infection Acute flaccid paralysis with OPV is extremely rare, and that when OPV is applied as a booster after the standard regimen with VIP vaccine, this risk is practically nil.”
The volume also released figures proving the safety of the polio vaccine. From 1989 to 2012, of the 764 million doses of oral polio vaccine given to children across the country, there were only 50 cases of polio vaccine in the country.
The case of flaccid paralysis in Pará is still under investigation by the Minister of State for health do Pará, the Ministry of Health and other diagnostic hypotheses, such as Guillain Barré syndrome, have not yet been ruled out. According to the ministry, the child was treated on an outpatient basis and was not hospitalized and is progressing well with the recovery of muscle strength. Another point emphasized by the volume is that the type of polio virus detected is not transmissible and does not change the epidemiological scenario in the national territory, since there have been no confirmed cases of polio since 1989.
Isabella Palalai explained that the investigation that will be conducted also includes determining the sequence of the polio virus present in the child’s stool. “The fact that the vaccine virus is present in the stool of a child who has received an oral vaccine does not mean that she has flaccid paralysis caused by the virus. In order to have this response, it is necessary to investigate other causes, and to sequence this virus to see if it is the virus of that vaccine.” , According to him. “We are still waiting to sequence this virus to give it a name and a nickname.”
The doctor adds that cases of paralysis caused by the vaccine virus are usually associated with conditions such as malnutrition and immunosuppression, and there is no risk of outbreak due to the discovery of the polio virus. “There is no need to panic, what we have to do is vaccinate children under five, and update the calendar for them.”
vaccination coverage
For the Head of the Infectious Diseases Department of the Brazilian Society of Pediatrics, Marco Aurelio Savade, regardless of the outcome of the investigation into the case in Pará, it is necessary to make it clear to parents and guardians of children that the polio vaccination schedule is safe and must be fully adhered to.
said Safadi, who highlighted the safety of the oral vaccine and its role in preventing tens of thousands of polio cases. “Even at a time when only this vaccine was being vaccinated, [o vírus vacinal] It was a very rare event, one that occurs once in every one or two million children who have been vaccinated. The vaccine is so safe that it has eradicated the disease here in the country for more than 30 years.”
observatory coordinator health In Infância (Observa Infância) of the Oswaldo Cruz Foundation (Fiocruz), Patrícia Boccolini said that the oral vaccine has an important contribution to the public health system and those responsible for it should not fear.
“VIP and VOP both have a purpose. VOP is so important to us, it’s important on our calendar,” he says. “Here in Brazil, we think from our point of view that VOP is practical. It does not need any professional with a lot of experience to apply it, because it drops. It is also easy to transport, easy to cool. So many advantages and why are countless reasons why we still apply” .
Researcher Fucruz asks that parents do not fail to immunize children at all doses stipulated in the PNI calendar, because Brazil is in a worrying situation, with low vaccine coverage for various diseases.
According to the National Immunization Program Information System (SI-PNI), projected doses of inactivated polio vaccine reached the target of 95% of the target audience for the last time in 2015, when coverage was 98.29% of children born that year.
After 2016, coverage fell to less than 90%, reaching 84.19% in 2019. In 2020, the COVID-19 pandemic affected the coverage of many vaccines, and this immunization reached only 76.15% of children. In 2021, the ratio was below 70% for the first time, 69.9%. In Para, where suspicion was registered, the percentage was even lower, 55.73%.
The problem is not limited to Brazil and Pan American only health The country and seven other countries in Latin America were listed as high-risk areas for polio re-introduction.

“Wannabe internet buff. Future teen idol. Hardcore zombie guru. Gamer. Avid creator. Entrepreneur. Bacon ninja.”